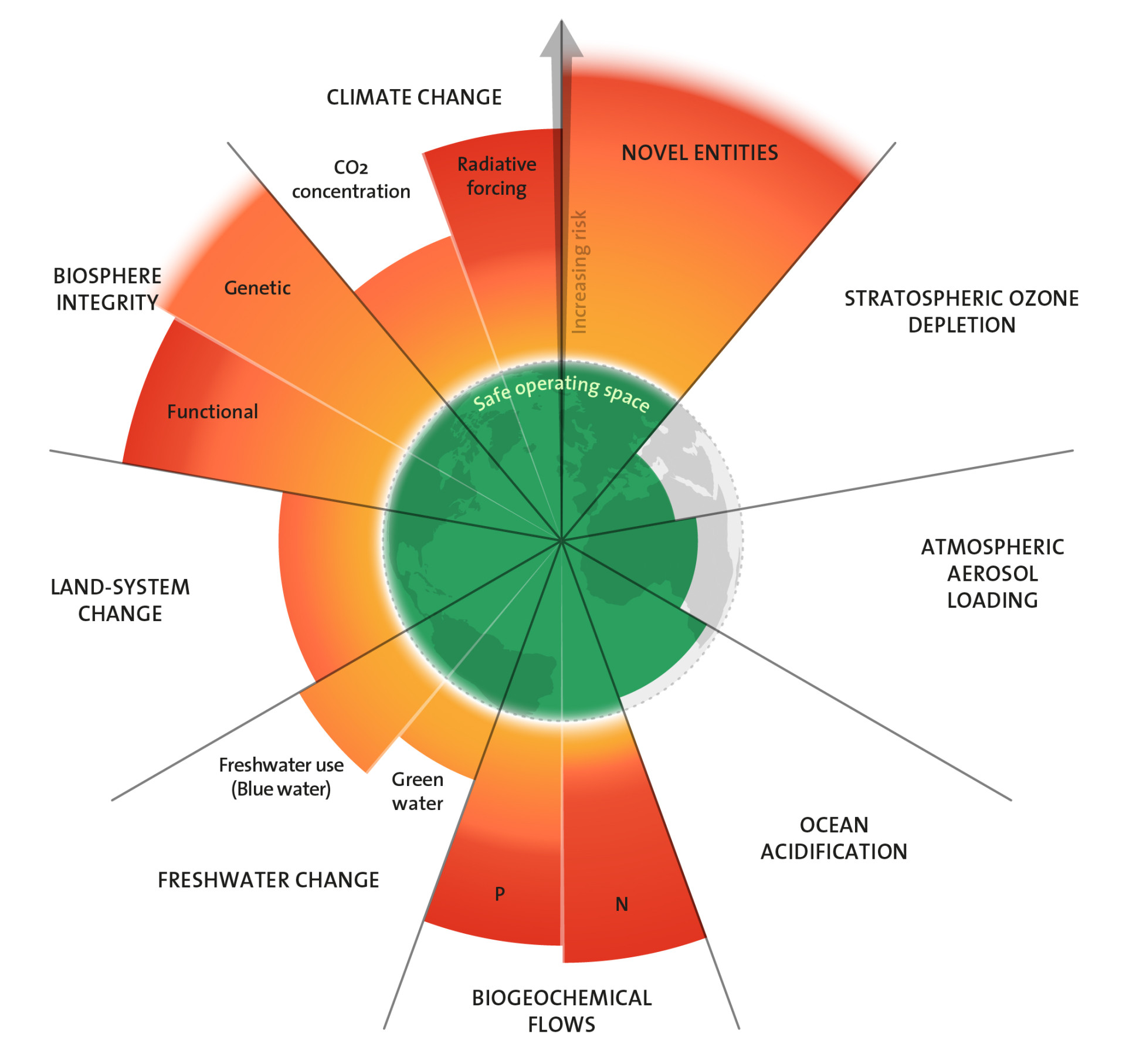
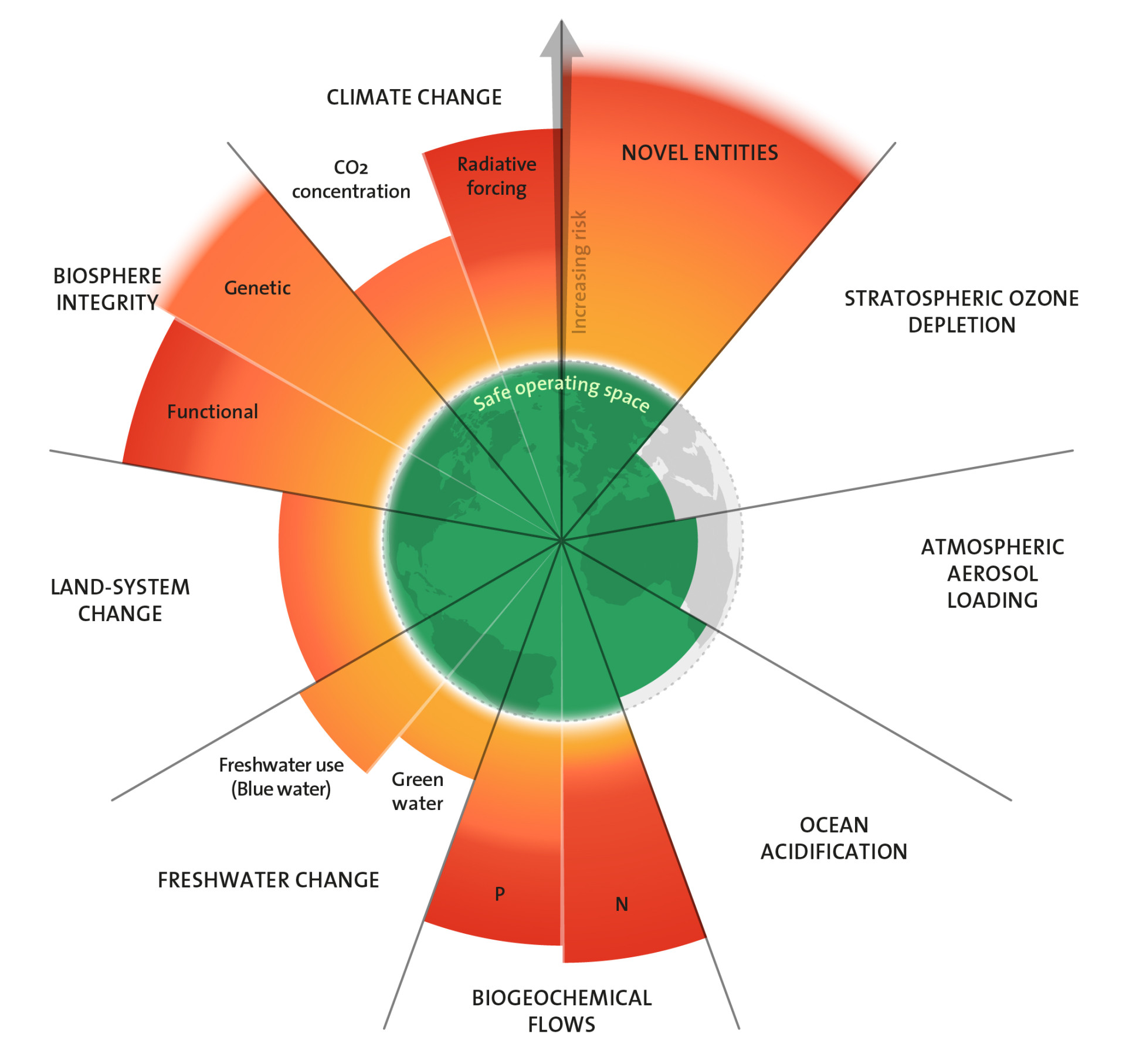
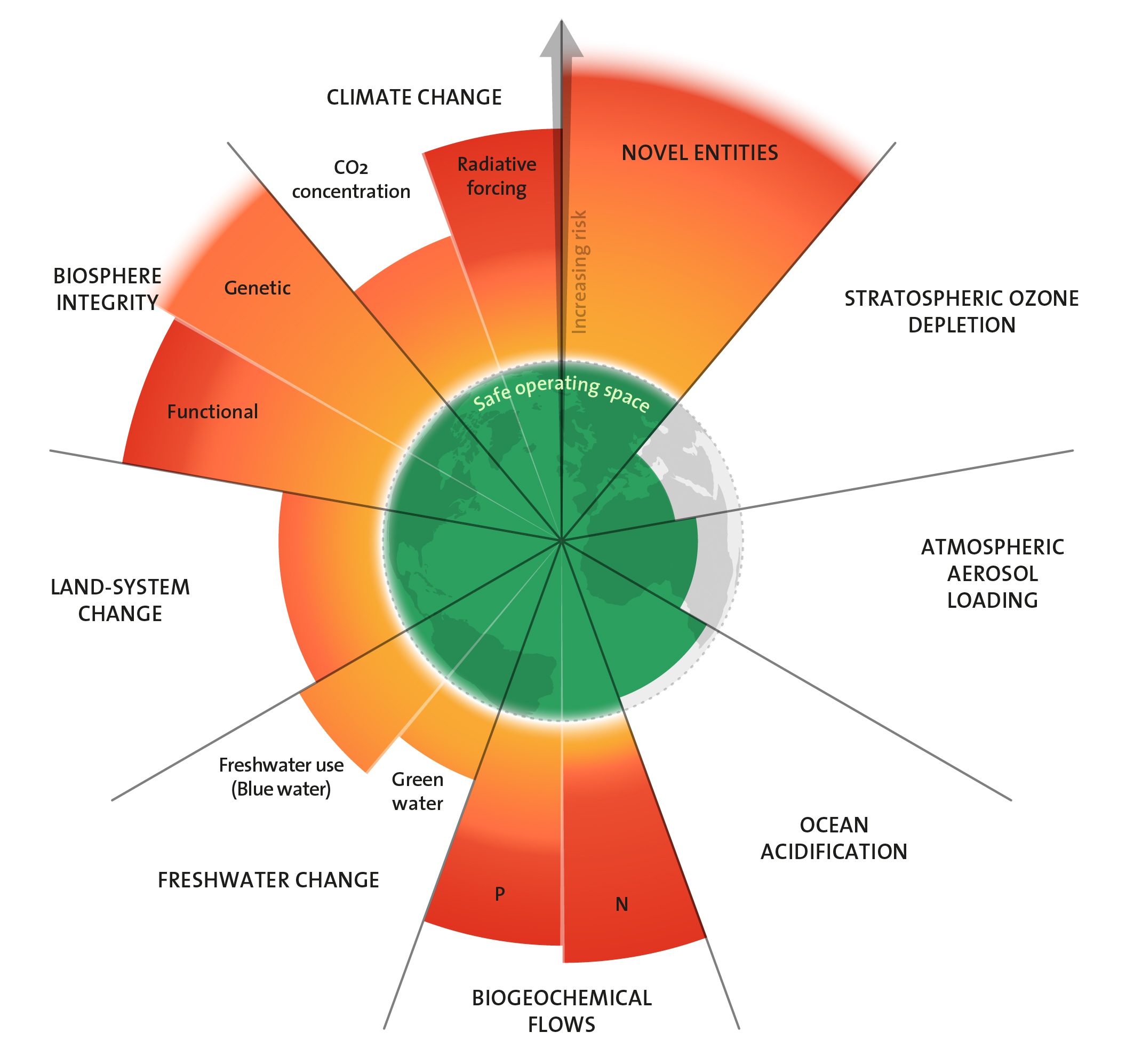
Planetary boundaries: balancing nutrient flows


Life could not exist without nitrogen and phosphorus. Like other important nutrients, these chemical elements circulate in cycles between land, water, air and living things – in quantities that ecosystems have adjusted to over the course of evolution. But humans have caused serious imbalances in these cycles. There is too much nitrogen and phosphorus in circulation. At the same time, there is a growing shortage of phosphorus.
Zu den essentiellen Elementen für alle Lebewesen gehören Stickstoff und Phosphor. Stickstoff ist essentieller Bestandteil des Erbguts und von Aminosäuren, aus denen alle Proteine aufgebaut sind. Phosphorverbindungen dienen im Stoffwechsel von Mikroorganismen, Pflanzen und Tieren unter anderem als wichtige Energieträger. In der Natur stehen allen Lebewesen begrenzte Mengen dieser essentiellen Stoffe zur Verfügung. Biologische Gleichgewichte sind darauf ausgerichtet, limitierte Nährstoffe optimal auszuschöpfen. Arten, Artengemeinschaften und Ökosysteme haben ihren Bedarf daran angepasst.
Verfügbarer Stickstoff verdoppelt
Die einzigen Lebewesen, die auf natürlichem Weg Stickstoff aus der Luft fixieren können, sind Bakterien. Meistens leben sie an den Wurzeln bestimmter Pflanzen wie Erbse oder Lupine, an die sie den Stickstoff abgeben. Aber auch aquatische Cyanobakterien (Blaualgen) können Stickstoff fixieren. In Form von Futter- und Nahrungsmitteln steht er dann Tier und Mensch zur Verfügung. Andere Mikroorganismen geben bei der Zersetzung organischen Materials elementaren Stickstoff wieder in die Atmosphäre ab.
Der Mensch jedoch führt ganz neue Dimensionen umgesetzter Stickstoffmengen in den Kreislauf ein. Chemiker:innen erfanden Anfang des 20. Jahrhunderts ein Verfahren, gasförmigen Stickstoff aus der Luft in eine Form umzuwandeln, die für Pflanzen verwertbar ist. Der Kunstdünger war erfunden. Für die Menschheit schien ein Segen. Die Bevölkerung wuchs und die Landwirtschaft war gefordert, Nahrungsmittel für alle bereitzustellen. Die Mengen an Stickstoff, die seither als Kunstdünger auf landwirtschaftlichen Flächen ausgebracht wurden und werden, liegen deutlich über den Mengen, die auf natürlichem Weg aus der Luft fixiert werden. Wissenschaftler:innen gehen davon aus, dass menschliche Aktivitäten in den letzten hundert Jahren die Menge an verfügbarem Stickstoff im Umlauf in etwa verdoppelt haben.
Kreisläufe verzerrt
Und nicht nur die Menge sprengt die natürlichen Grenzen. Zudem verzerrt der Mensch den Stickstoffkreislauf regional und weltweit. Die Nachfrage nach Agrarprodukten trägt dazu weiter maßgeblich bei. Zum Beispiel wird Soja, eine der Pflanzen, die Stickstoff von Bakterien aufnimmt, als Futtermittel vom amerikanischen Kontinent nach Europa importiert. Der verfütterte Stickstoff gelangt als Fleisch teilweise auf die europäischen oder als Export auf weltweite Märkte. Rund um die Standorte der Tierhaltung wird ein erheblicher Teil in Form von Gülle aus den Ställen zum Düngen auf die Felder aufgebracht.
Stickstoff aus Gülle und Kunstdünger, den die Pflanzen nicht aufnehmen, sickert von dort ins Grundwasser oder wird ausgewaschen und belastet Grundwasser, Flüsse und Seen sowie die Küstenzonen der Meere. Auch Stickstoff aus Industrie- und Verkehrsabgasen gelangt zum Teil mit dem Regen in Böden und Gewässer.
Schäden zu Land, zu Wasser und in der Luft
Zu den Folgen der Überdüngung gehört das zeitweise massive Wachstum bestimmter Algen. In Kombination mit einer Erwärmung des Wassers führt es häufig zu Sauerstoffmangel, den im schlimmsten Fall kaum Tiere und Pflanzen überleben. Das Gewässer verödet, es ‘kippt um’. Auch an Land überwuchern schnell wachsende Pflanzen solche, die eigentlich an nährstoffärmere Bedingungen angepasst sind. Der Überschuss an leicht verfügbaren Nährstoffen engt das Artenspektrum ein und bringt ganze Ökosysteme aus ihrem Gleichgewicht.
Auch in die Luft geben überdüngte Agrarflächen Stickstoff ab: Gasförmiges Ammoniak kann Ökosysteme durch eine Überversorgung mit Nährstoffen schädigen und trägt zur Versauerung von Böden bei. Bis in die Atmosphäre sind die Folgen des menschlichen Umgangs mit Stickstoff zu spüren, denn Agrarflächen geben die Stickstoffverbindung Lachgas in die Luft ab. Das starke Treibhausgas trägt zur Erderwärmung bei und beeinflusst die Bildung und den Abbau von Ozon. Industrielle Verbrennungsprozesse und Treibstoffverbrauch im Verkehr setzen weitere gasförmige Stickstoffverbindungen aus fossilen Energieträgern frei, die in der Atmosphäre ihre schädliche Wirkung entfalten. Stickstoffoxide aus Flugzeugabgasen greifen dort zum Beispiel die schützende Ozonschicht an.
Beschleunigte Freisetzung von Phosphor
Ähnlich wie Stickstoff ist Phosphor ein essentielles Nährelement für Pflanzen. Wasserlösliche Phosphorverbindungen werden als Düngemittel auf vielen landwirtschaftlichen Flächen ausgebracht. Auch sie verursachen Algenwachstum und Sauerstoffmangel in Gewässern. Der Grundstoff für die Düngemittel sind fossile Lagerstätten. Der Mensch setzt binnen kürzester Zeit Mengen an Phosphorverbindungen in die Umwelt frei, die sich über Millionen von Jahren gebildet haben und unter der Erde lagerten.
Industrialisierung und Intensivierung der Landwirtschaft haben die globalen Einflüsse des Menschen auf die natürlichen Stoffkreisläufe verstärkt. Den Vergleichswert aus dem vorindustriellen Zeitalter für die Menge an fixiertem, in Umlauf gebrachtem und im Kreislauf umgelenkten Stickstoff und Phosphor, setzen die Autoren der Planetaren Grenzen auf null. Wissenschaftler:innen versuchen zu ermitteln, wie weit der Mensch in die Kreisläufe eingreifen kann, ohne die Stabilität der Ökosysteme und fundamentaler geophysikalischer und geochemischer Prozesse zu gefährden.
Grenzen weit überschritten
Für Phosphor betrachten sie regionale und globale Stoffströme und definieren zwei Grenzen. Die erste legt die Menge an Phosphor fest, die maximal als Dünger auf Böden freigesetzt werden soll. Die Autor:innen nennen dies die regionale Grenze und haben 2015 einen eigenen Grenzwert hierfür festgesetzt. Durch Bodenerosion und Wasserabfluss von Agrarflächen gelangen Teile davon in Seen und Flüsse und schließlich ins Meer. Als Maß für die weltweiten Phosphorströme betrachten sie deshalb als Zweites die Gesamtmenge des Elements, die in die Meere eingetragen wird. Derzeit sind die Grenzen für Phosphorfreisetzung sowohl auf regionaler wie auf globaler Ebene um ein Vielfaches überschritten.
Für Stickstoff begrenzt das Konzept die Menge, die durch industrielle und landwirtschaftliche Prozesse aus der Luft fixiert und verfügbar gemacht werden darf, um in den sicheren Handlungsraum eines stabilen Lebensraumes für den Menschen und andere Arten zurückzukehren. Um sich die Größenordnungen vor Augen zu führen: Etwa 100 bis 120 Millionen Tonnen industriell fixierten Stickstoffs werden pro Jahr als Dünger auf Agrarflächen ausgebracht. Hinzu kommt die vom Menschen gezielt herbeigeführte Stickstofffixierung durch Bodenbakterien in Verbindung mit Kulturpflanzen wie Soja. Die Menge liegt in der Größenordnung von 30 bis 100 Millionen Tonnen pro Jahr. Zusammengenommen führen menschliche Aktivitäten dem Kreislauf jedes Jahr also etwa 130 bis 220 Millionen Tonnen Stickstoff in Formen zu, die für Pflanzen verfügbar sind. Die weltweite Grenze haben die Forschenden auf 62 Millionen Tonnen pro Jahr gesetzt. Sie ist also um das Zwei- bis Dreifache überschritten.
Was können wir tun, um in die Grenzen zurückzukehren?
Die Planetaren Grenzen sind weltweite Durchschnittswerte, an denen die Menschheit sich orientieren kann. Für die Auswirkungen der Umverteilung natürlicher Elemente ist jedoch die regionale Verteilung ebenso entscheidend wie ihre Gesamtmenge im Umlauf.
Unter dem Aspekt sind heutige Praktiken der Lebensmittelindustrien – global verschiffte Futtermitteltransporte und lokal konzentrierte Tierhaltung sowie teilweise übermäßige Düngung mit Stickstoff und Phosphor – kritisch zu sehen. Wissenschaftler:innen sprechen sich für Formen der Landwirtschaft aus, bei denen weniger Tiere pro Fläche gehalten und Düngemittel effizienter eingesetzt werden. Dabei stellt sich die drängende Frage, wie wir gleichzeitig die Nahrungsmittelsicherheit für weite Teile der globalen Bevölkerung gewährleisten können.
Denn weltweit reduzierter Düngemitteleinsatz führt zu Verknappungen des globalen Angebots. Die in der Folge steigenden Preise würden eine Verschlechterung der Ernährungssituation in armen Ländern bedeuten.
Detailliertere Kenntnisse der regionalen und globalen Stoffkreisläufe, neuartige Techniken und angepasste Praktiken in der Landwirtschaft können stellenweise helfen, Stickstofffreisetzung aus dem Anbau zu reduzieren. Dabei müssen jedoch Verlagerungseffekte konsequent mitgedacht werden: Die weltweite Freisetzung von Stickstoff und Phosphor wird nicht weniger, wenn in deutschen Ställen weniger Gülle produziert, dafür aber Fleisch aus anderen Ländern importiert wird. Deshalb erfordert eine nachhaltige Umstellung der Agrarproduktion ein verändertes Bewusstsein der Verbraucher:innen und der Politik. Nachfrage und Verbrauch landwirtschaftlicher Produkte müssen sich an den Grenzen des Planeten orientieren und entsprechend reguliert werden. Neben der Landwirtschaft sind Verbrennungsabgase aus Verkehr und Industrie ein wichtiger Aspekt: Sie müssen reduziert werden. Die Verkehrswende ist ein wichtiger Pfad zu diesem Ziel.
Fachliche Prüfung und Beratung zu diesem Beitrag: Prof. Dr. Doris Vetterlein, UFZ; Prof. Dr. Hans-Jörg Vogel, UFZ; PD Dr. Reinhard Well, Thünen Institut für Agrarklimaschutz
Zurück zur Übersichtsseite Planetare Grenzen
Expertise