Planetary boundaries: how we saved the ozone layer


The ozone layer protects all forms of life on Earth from hazardous ultraviolet radiation. In 1985, scientists noticed that this protective shield had become considerably thinner over the Antarctic. The cause of what became known as the “ozone hole” was chlorofluorocarbons (CFCs), a group of chemicals that can also be harmful to the climate. The international community acted quickly and succeeded in phasing them out. Today the ozone layer is gradually recovering.
Licht und Wärme der Sonne ermöglichen Leben auf der Erde. Das Spektrum der Sonnenstrahlung umfasst aber auch für Menschen unsichtbares, sehr energiereiches ultraviolettes (UV-) Licht. Trifft es ungefiltert auf die Erdoberfläche, kann es das Erbgut von Menschen, Tieren, Pflanzen und Mikroorganismen schädigen. Menschen und Tiere erleiden vor allem Schäden an Augen und Haut. Zudem bremst UV-Strahlung die Photosynthese, den Prozess, in dem Pflanzen CO2 binden und Sauerstoff produzieren. Vor den gefährlichen kurzwelligen UV-Strahlen schützt uns die natürliche Ozonschicht die etwa 15 bis 50 Kilometer über unseren Köpfen in der Stratosphäre liegt. Doch Abgase und Klimawandel gefährden den Schutzmantel der Erde.
Ozonloch über der Antarktis
In den 1980er Jahren machte das Ozonloch Schlagzeilen. Verursacht durch Fluorchlorkohlenwasserstoffe (FCKW), die als Treib- und Kühlmittel eingesetzt wurden, breitete es sich in jedem Frühling über der Antarktis aus. Entscheidend dafür sind die dort herrschenden niedrigen Temperaturen. Bei unter -78°C bilden sich die Polaren Stratosphärenwolken. Diese besonderen Wolken schaffen eine Oberfläche, auf der Abbauprodukte der FCKW bei Sonneneinstrahlung die Ozonschicht angreifen. Der winterliche Polarwirbel hält die polaren Luftmassen zusammen. Regelmäßig in den Monaten des polaren Frühjahrs dehnt sich das Loch in der Ozonschicht über der Südpolarregion aus. In den Sommermonaten bricht der Polarwirbel zusammen und einströmende ozonreichere Luftmassen aus den mittleren Breiten sorgen dafür, dass die Schicht sich wieder verdichtet.
Treffen die kurzwelligen UV-Strahlen Menschen und Tiere, fördern sie die Trübung der Linse. Schafzüchter in Südamerika berichteten in den 1980er Jahren von erblindeten Tieren. Auch bei Fischen wurde der Effekt beobachtet. Bei Menschen verursachen die Strahlen den grauen Star, fördern Sonnenbrand und begünstigen die Entstehung von Hautkrebs. Pflanzen verlieren durch hohe UV-Einstrahlung ihre Fähigkeit, CO2 aufzunehmen, Biomasse zu produzieren und Sauerstoff abzugeben. Die Nahrungsgrundlage der Menschen schien grundsätzlich gefährdet, würde sich das Ozonloch weiter ausbreiten.
Langfristige Nachwirkungen
Die Forschung und das Engagement von Wissenschaftler:innen um den 2021 verstorbenen Paul Crutzen trugen damals maßgeblich dazu bei, dass 1987 das Montrealer Protokoll verabschiedet wurde: FCKW wurden weltweit geächtet. Dieses bedeutende völkerrechtliche Abkommen führte dazu, dass die Neuproduktion der menschengemachten Ozonkiller schnell reduziert und Alternativen entwickelt wurden. Doch die FCKW-Moleküle in der Atmosphäre sind sehr langlebig. Ihre Abbauprodukte wie Chlor greifen noch Jahrzehnte nach der Freisetzung die Ozonschicht an. So dauerte es bis ins 21. Jahrhundert, ehe die Ausdehnung des Ozonlochs nicht mehr zunahm. Etwa seit dem Jahr 2000 geht die Chlor-Konzentration in den entscheidenden Luftschichten langsam zurück. Wissenschaftler:innen erwarten für die zweite Hälfte dieses Jahrhunderts, dass die Ozonschicht ihre Dichte von 1980 wieder erreicht.
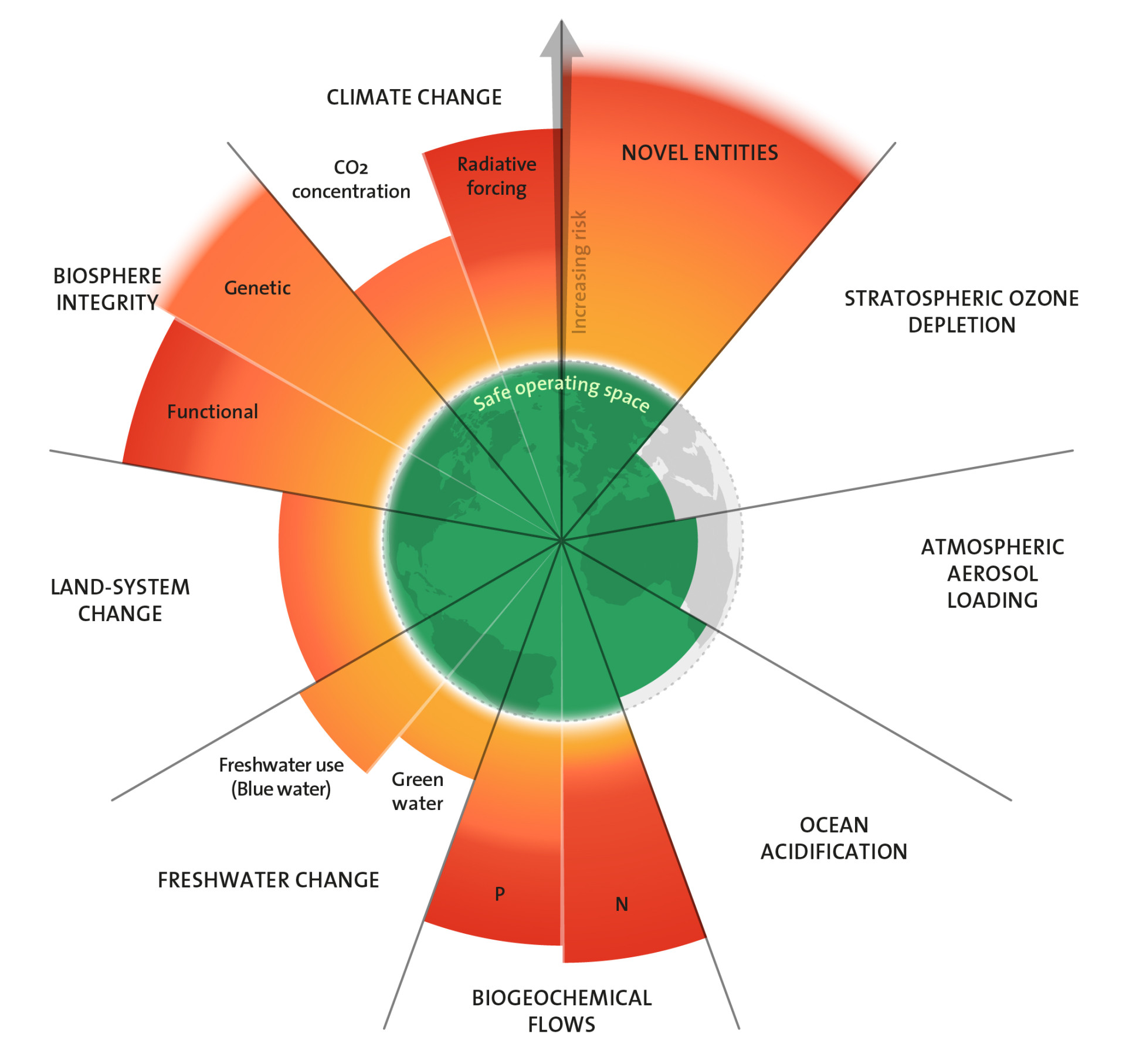
Je nach Breitengrad ist die Ozonschicht über der Erde unterschiedlich dicht und dick. Das hat zum einen natürliche Gründe, die in den Mechanismen der Ozonbildung und in weltweiten Luftströmungen liegen. Zum anderen wirkt der Effekt der FCKW wie beschrieben insbesondere in der Kälte der polaren Stratosphäre. Das Konzept der Planetaren Grenzen sieht für die Ozonschicht einen weltweit einheitlichen Grenzwert vor: Ihre Stärke soll im globalen Mittel mindestens 95 Prozent der Stärke im vorindustriellen Zeitalter betragen. „Über den Sinn so einer einheitlichen Grenze kann man diskutieren“, sagt Jens-Uwe Grooß, Stratosphärenforscher am Forschungszentrum Jülich. Denn in der Südpolarregion wird dieser Zielwert zwar jedes Jahr unterschritten. Im globalen Durchschnitt liegt der Wert jedoch darüber, wenn auch (noch) nicht auf der Höhe des vorindustriellen Vergleichswertes. Sind wir damit jetzt auf der sicheren Seite?
Neue Gefahren durch Klimawandel
Nicht ganz. Tendenziell wird das Ozonloch zwar seit fast 20 Jahren kleiner. Ein Teil der vor Jahrzehnten freigesetzten FCKW und ihrer Abbauprodukte, etwa Chlor, verbleiben aber bis heute in der Atmosphäre. Hinzu kommen neuere Einflüsse auf die Dynamik der Ozonschicht: Aufgrund des Klimawandels können zum Beispiel Waldbrände häufiger auftreten und riesige Flächen betreffen. Aufsteigender Ruß aktiviert verbliebene Chlormoleküle, sodass verstärkt Ozon abgebaut wird. Große Waldbrände können die Regeneration der Ozonschicht deshalb verzögern.
Klimawandel fördert Ozonabbau in der Arktis
Auch über der Arktis ist die Ozonschicht ausgedünnt. Forschende der MOSAiC-Expedition bestätigten im Frühjahr 2020 den signifikanten Ozonverlust in den Wintermonaten der Nordpolarregion. Auswertungen der Daten, die während der Expedition gesammelt wurden, ergaben: Der Trend zu höheren Ozonverlusten in den polaren Wintermonaten geht mit dem Klimawandel einher. Dieselben Treibhausgase, die zur weltweiten Erwärmung führen, sorgen für eine Abkühlung stratosphärischer Luftschichten. So verstärken sie auch in der Nordpolarregion den Ozonabbau im Frühjahr. Forschende unter anderem des Alfred-Wegener-Instituts für Polar- und Meeresforschung gehen davon aus, dass der saisonale Verlust von Ozon bis zum Ende dieses Jahrhunderts weiterhin auftreten wird und sogar noch schlimmer werden könnte. Die Folgen zeigen sich zeitweise bis in unsere Breiten. „Der arktische Polarwirbel driftet immer mal wieder über Mitteleuropa, sodass es auch in Deutschland jeweils im Frühjahr zu einigen Tagen reduzierter Ozonschicht kommen kann“, erklärt der Polarforscher und MOSAiC-Expeditionsleiter Markus Rex.
Und nicht alle Schäden an der weltweiten Ozonschicht gehen auf FCKW zurück. Auch Stickstoffverbindungen beeinflussen die Entstehung und den Abbau von Ozon in verschiedenen Luftschichten. Beispielsweise werden Einflüsse von Lachgasemissionen aus der Landwirtschaft auf die Dichte der Ozonschicht diskutiert. „Die Dynamiken in der Atmosphäre verändern sich im Zuge des Klimawandels in vieler Hinsicht“, fasst Jens-Uwe Grooß zusammen. „Auch wenn wir im Mittel eine Dichte der Ozonschicht wie im vorindustriellen Zeitalter erreichen, wird sie regional andere Eigenschaften haben als 1980 oder 1850.“
Was können wir tun?
Damit die Ozonschicht vollständig heilen und sie ihre natürliche Schutzfunktion erfüllen kann, müssen wir also nicht nur das – bisher erfolgreiche – Montrealer Protokoll strikt einhalten. Vielmehr gilt es, die komplexen Zusammenhänge weiter zu erforschen, die sich zwischen den verschiedenen Einflüssen des Menschen auf alle Sphären des Planeten entfalten. Der Klimawandel mit seinen vielfältigen, zum Teil noch unerforschten Auswirkungen ist vielleicht die größte Herausforderung, der die Menschheit jemals gegenüberstand. Die Erfolge des Montrealer Protokolls können uns Hoffnung machen, und beispielhaft Wege weisen, wie wir als Weltgemeinschaft Umweltprobleme konstruktiv bewältigen können.
Fachliche Prüfung und Beratung zu diesem Beitrag: Dr. Jens-Uwe Grooß, Forschungszentrum Jülich
Zur Übersichtsseite Planetare Grenzen