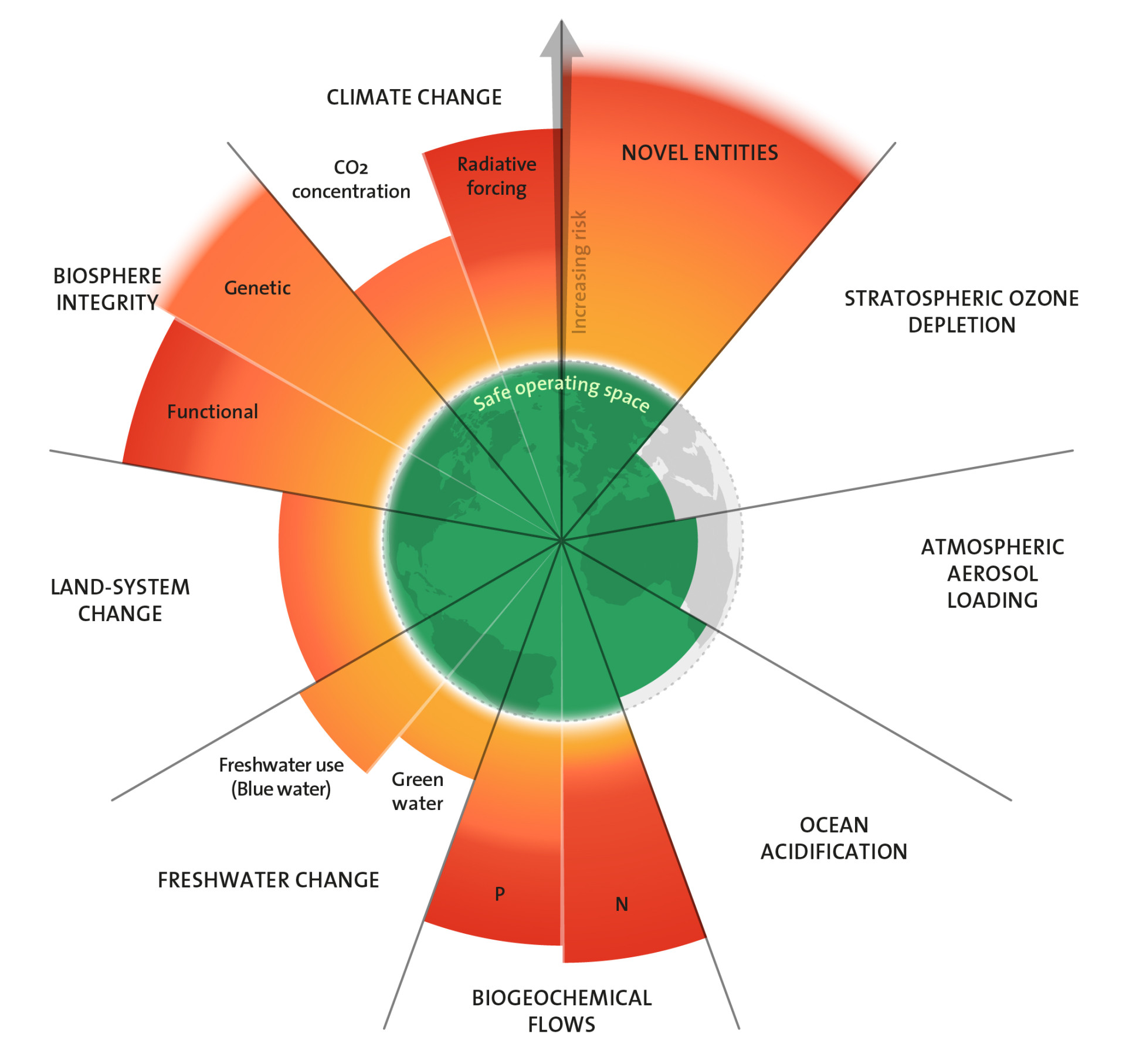
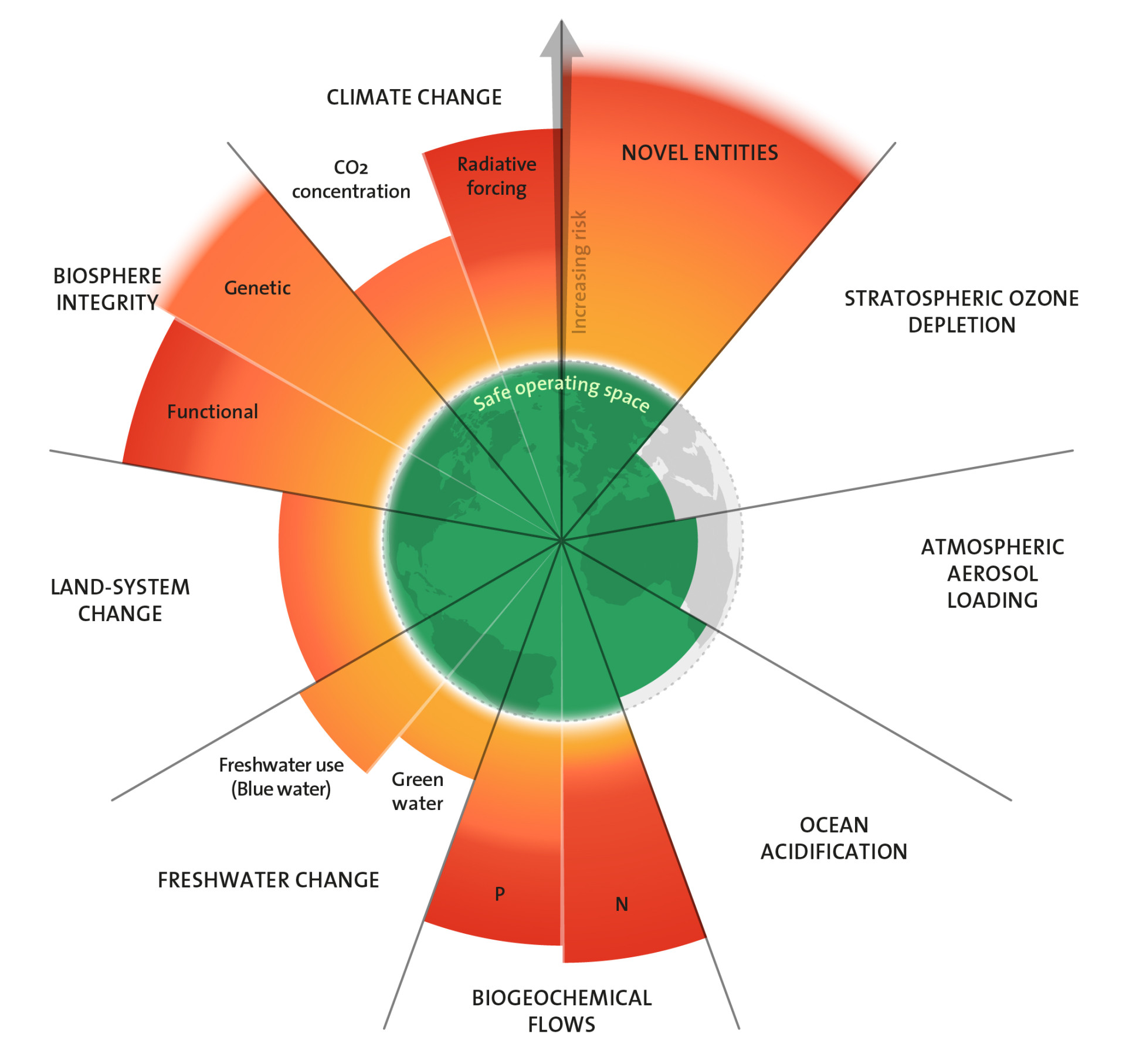
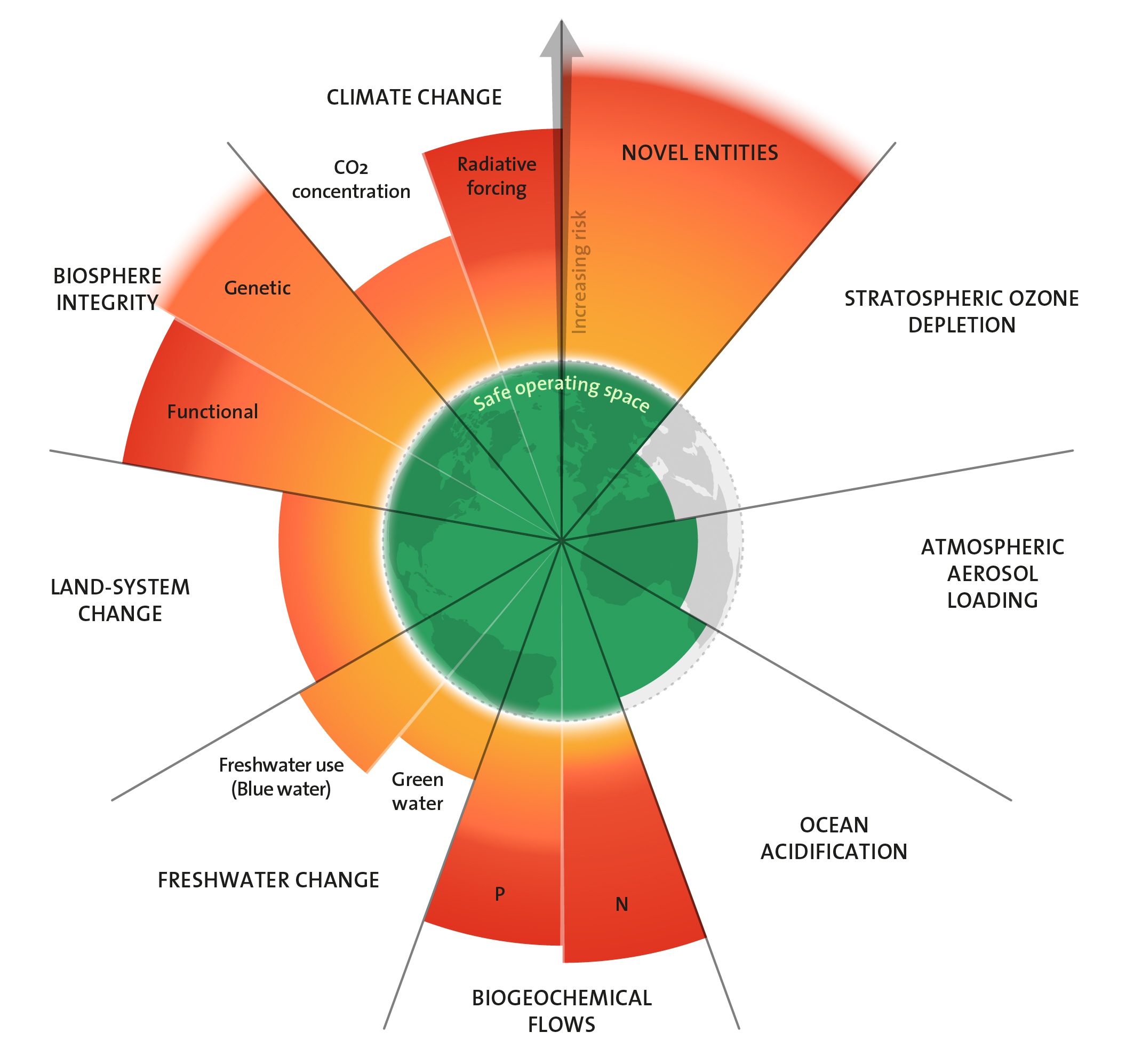
Planetary Boundaries: Oceans are becoming more acidic


Oceans are becoming more and more acidic; their pH value is decreasing. This is because of more and more carbon dioxide entering the atmosphere, which dissolves in oceanic water, turning into carbonic acid. This phenomenon is impacting sea creatures and marine ecosystems, which in turn will have consequences for human beings. However, the planetary boundary for acidification has not yet been exceeded.
Das massenweise Verheizen fossiler Brennstoffe setzt binnen kürzester Zeit Kohlenstoffdioxid frei, das Jahrmillionen unter der Erde lagerte. Der Gehalt an Kohlenstoffdioxid (CO2) in der Atmosphäre beträgt derzeit im weltweiten Mittel rund 420 ppm (Parts per Million). Die Konzentration liegt damit über 40 Prozent höher als vor der Industrialisierung. Der Anstieg des atmosphärischen CO2 ist der Haupttreiber des globalen Klimawandels. Und die Folgen des menschlichen Eingriffs in die Kohlenstoffverteilung wären noch viel dramatischer, würden nicht die Ozeane als Puffer dienen: Sie nahmen rund ein Viertel bis ein Drittel des seit 1750 ausgestoßenen CO2 auf.
Meeresökosysteme leiden
Diese Pufferleistung geht allerdings auf Kosten der Ökosysteme in den Meeren. Denn nicht nur das Klima gerät durcheinander. Das CO2 bildet im Wasser Kohlensäure, die Ozeane werden immer saurer. Der pH-Wert des Meerwassers ist mit dem Anstieg von CO2 in der Luft in den vergangenen Jahrzehnten rapide gesunken. Diese Versauerung führt im Meerwasser zum Abbau von gelöstem Karbonat, einem unverzichtbaren Baustoff für manche freischwebende Kleinstlebewesen (Plankton) und vor allem für aquatische Schalentiere wie Muscheln und Schnecken sowie für Korallen. Plankton bildet die Grundlage der Nahrungskette in vielen Meeresökosystemen. Muscheln haben neben ihrer Rolle in den Nahrungsnetzwerken wichtige Filter- und Reinigungsfunktionen. Und Korallenriffe bilden einzigartige Lebensräume für eine große Vielfalt von Tieren und Pflanzen.
Schmaler Grat mit schlechter Aussicht
Das Konzept der Planetaren Grenzen sieht die Menschheit in einem sicheren Handlungsspielraum, solange die Karbonatkonzentration im Oberflächenwasser der Ozeane im Durchschnitt bei mindestens 80 Prozent der vorindustriellen Konzentration liegt. Derzeit bewegen wir uns auf einem schmalen Grat entlang dieser Grenze. Gleichzeitig mehren sich wissenschaftliche Befunde, dass schon die derzeitigen Bedingungen für bestimmte Meeresorganismen lebensbedrohlich sind. Auch scheinen einige Arten empfindlicher auf den sinkenden pH-Wert zu reagieren, als bisher bekannt war. Und die Aussichten für die Ozeane sind schlecht: Der Trend der Versauerung verstärkt sich, da die Menschheit noch immer mehr statt weniger CO2 emittiert. Fachleute diskutieren unterdessen aufgrund des aktuellen Wissensstandes, den Grenzwert für die Versauerung der Meere anzupassen.
Neben der Versauerung ist die Erwärmung der Ozeane durch den Klimawandel ein wichtiger Faktor. Sie verändert die Aufnahmekapazität des Wassers für CO2, wärmeres Wasser nimmt weniger CO2 auf. Zudem verändert der Temperaturanstieg wichtige Stoff- und Energie-Transportströme zwischen Oberfläche und tieferen Schichten der Ozeane. Sie werden daher künftig nicht mehr im bisherigen Maß als Kohlenstoffsenke zur Verfügung stehen. Die verschiedenen Mechanismen dahinter greifen auf komplexe Art und Weise ineinander. Deshalb ist es schwierig, präzise Vorhersagen über Art und Ausmaß der Änderungen zu machen.
Was können wir tun?
Der Zustand der Meere ist kritisch. Auch wenn die Forschenden im Update der Planetaren Grenzen von 2023 davon ausgingen, dass die Grenze der Ozeanversauerung gerade noch eingehalten wird, dürfen wir uns nicht in Sicherheit wägen. CO2-Emissionen so weit wie möglich zu senken ist wichtiger und drängender denn je. Unvermeidbare Emissionen müssen aus der Atmosphäre entfernt und unschädlich gemacht werden. Dabei helfen Maßnahmen zur nachhaltigen Aufforstung sowie als letzte Möglichkeit neue Technologien. Eine Strategie, die bei der Entwicklung verfolgt wird, ist die direkte Abscheidung von CO2 aus der Luft. Eine andere vielversprechende Methode, um CO2 aus der Atmosphäre zu binden ist, den pH-Wert der Ozeane zu erhöhen. Dazu werden in Forschungsprojekten verschiedene natürliche Mineralien getestet, die dem Meerwasser zugegeben werden könnten. Gelingt es, die Ozeane auf diesem Weg zu alkalinisieren, dämpft das den CO2-Anstieg in der Atmosphäre und gleichzeitig die Versauerung der Meere.
Related links
Weitere 8 Planetare Grenzen
Fachliche Prüfung und Beratung zu diesem Beitrag: Prof. Dr. Ulf Riebesell, GEOMAR
Zurück zur Übersichtsseite Planetare Grenzen